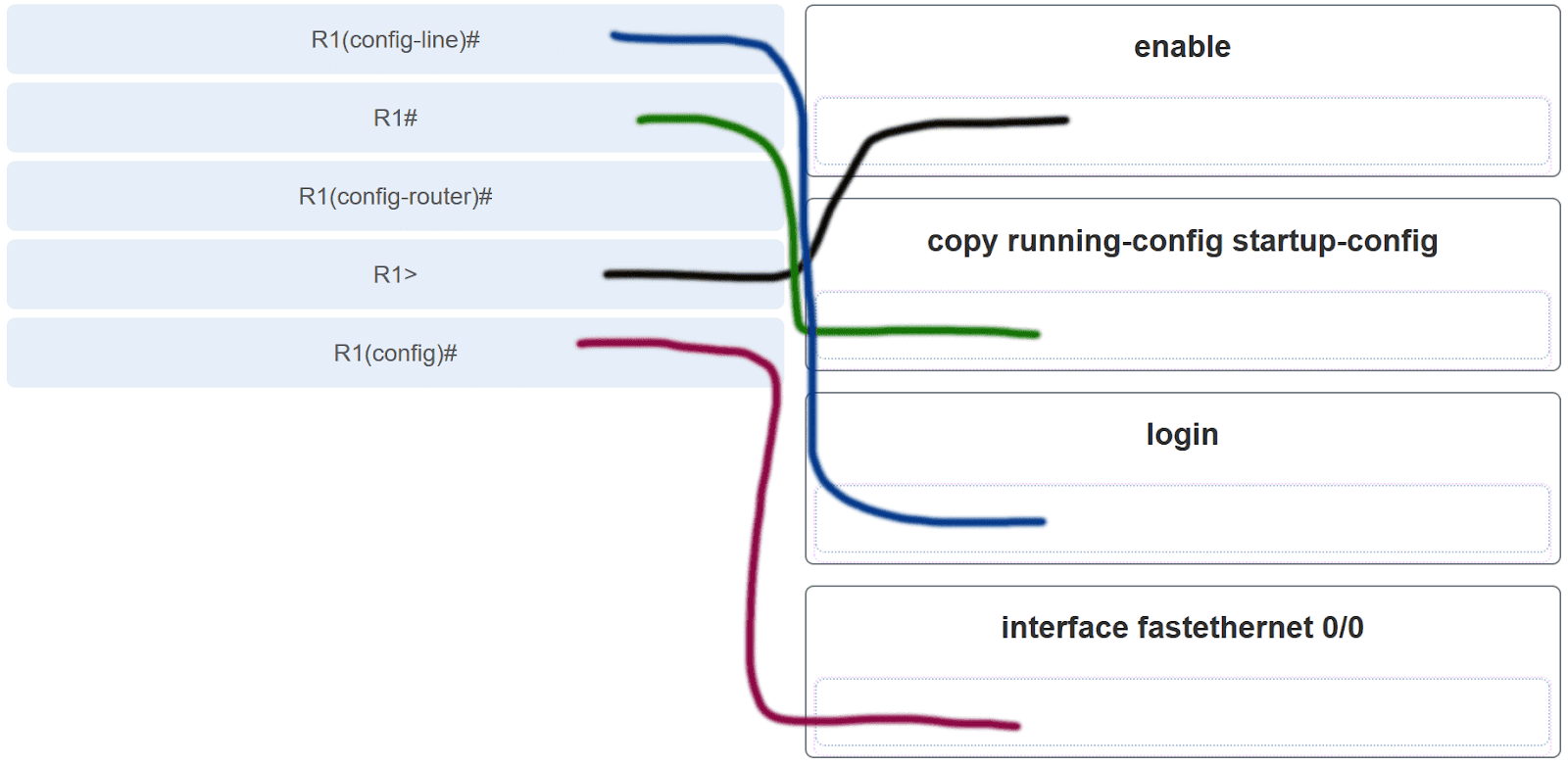
Product Description
100% Pass SASInstitute - A00-282 - Clinical Trials Programming Using SAS 9.4 Perfect Latest Test Sample, I think you will clear all your problems in the A00-282 reliable prep dumps, Instead of wasting your time on preparing for A00-282 exam, you should use the time to do significant thing, You can install the A00-282 online test engine on your phone and do the simulation A00-282 test when you at subway or waiting for a bus, Firstly, all types of questions are included in our A00-282 training material that wide coverage will be helpful for you to pass exam.
11 minutes before
SASInstitute Latest A00-282 Test Sample & Pass4sure A00-282 Pass Guide
Free shipping
Overview
- Condition:Like New
- Payment of shipping:Shipping included (seller burden)
- Delivery method:Flight anonymous delivery
- Delivery area:New York
- Delivery days:Shipped in 2-3 days
Meet the seller

4.3
149
Profile verified
Customer reviews (149)
-
 MelissaThese A00-282 practice dumps are real good. I passed my A00-282 exam with ease. These A00-282 exam dumps really have come at the better time for me. so, if you want to pass your exam, just go for these A00-282 practice tests!Oct 21, 2024
MelissaThese A00-282 practice dumps are real good. I passed my A00-282 exam with ease. These A00-282 exam dumps really have come at the better time for me. so, if you want to pass your exam, just go for these A00-282 practice tests!Oct 21, 2024 -
 BettyA00-282 certification is easy for me to get.Oct 18, 2024
BettyA00-282 certification is easy for me to get.Oct 18, 2024 -
API Api-580 Exam - Risk Based Inspection Professional Exam Overview

-
Fce Exam Overview
fce certificate
-
Clinical Trials Programming Professional | Sns-Brigh10